|
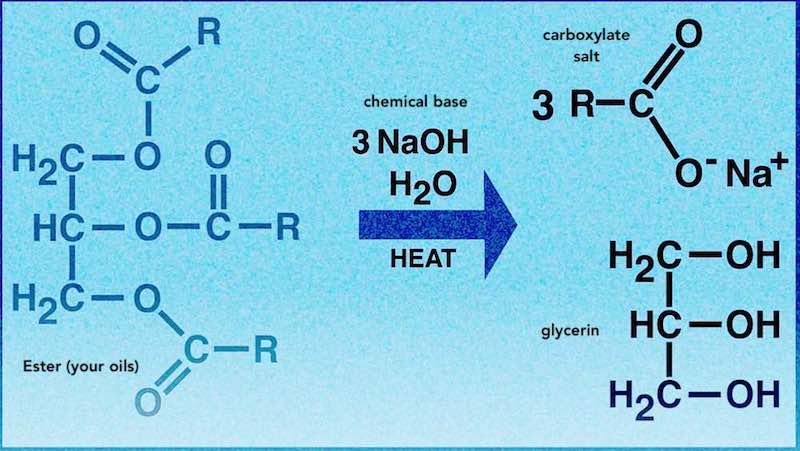
Do you know the chemistry behind making handmade soap? Do you sell it or give it to loved ones? Don't worry, we've got you! Handmade Soap | The Chemistry of Soap MakingThe Chemistry of SoapDo you understand the chemistry behind making your handmade soap? Do you sell it or give it to loved ones? Making handmade soap involves using some pretty dangerous chemicals and if used incorrectly you can really hurt someone. The chemicals used to make handmade cold process soap are particularly hazardous and can cause serious skin burns, irritation and allergic reactions if not handled properly. This situation quite rightfully scares beginners, as so it should! If you plan on selling, gifting or using your cold process soap and giving it to loved ones, you need to know what you are doing. In this 'Chemistry of Handmade Soap Making' series we are going to learn about the basic chemistry of making your own handmade soap, properly and safely. We are going to gently walk you through everything you need to know to fully understand the basic chemistry behind making handmade soap. We will learn about the ingredients, the chemistry behind the reactions and how to apply standard 'bench', or 'wet' chemistry, to making handmade soap. This will ensure that you fully understand what you are doing and that in the event of a claim against your newly formed handmade soap company, your insurance company (you are insured right?), will actually pay out! 1. Brief History of SoapA lot of sources will tell you that people stumbled upon soap in the process of some magical 'happening' thousands of years ago in a forest. That's not quite the case. Specifically what seems to have happened is that in the course of cooking animals on a wood burning fire, something akin to weak soap was formed inside the ashes. This soapy substance was found to clean things quite effectively and this appears to have been the discovery of soap. Quite whether this is the case is open to debate. However, if you have ever roasted a large animal on an open wood fire, you'll know that the the fat from the animal will drip into the fire. Depending on the type of wood, you will get potassium carbonate forming in the ashes. Potassium carbonate will break down the fat and when liquid is applied, you'll get a soap. Bingo! 2. Chemistry of Soap - Quick VersionSaponification in solid soap (cold and hot process soap) is a hydrolysis reaction that occurs when we combine a long chain fatty acid, that is, a fatty acid ester, with a chemical base and water. The result is soap and glycerin, or more specifically, a carboxylate salt and glycerin. So soap is in fact a salt. Solid soap is predominantly made using the chemical base sodium hydroxide while soft soap (liquid soap) is primarily made using the base potassium hydroxide. The chemical reaction is shown below. 3. Acids and BasesWe know something is an acid when it has one or more hydrogens in its chemical formula, for example, HCL (hydrochloric acid) and H20 (water). Acids are hydrogen donors. We know something is a base when it has an OH listed in its formula, e.g., NaOH (sodium hydroxide) or KOH (potassium hydroxide). Hydroxides are hydrogen acceptors. Acids are sour in taste and bases are bitter in taste and have a slippery, soapy feel.
Bases are commonly called alkali, alkaline, lye and caustic. Sodium hydroxide is often called caustic soda, potassium hydroxide is sometimes called caustic potash (remember the animal fat fire from earlier?) both are thus used in the production of soap. Acids and bases will both chemically burn skin and must be handled with extreme care! Acid burns can be felt relatively quickly but burns from a chemical base are often NOT felt immediately which makes them particularly dangerous.
1 Comment
Sandy Dean
7/1/2023 09:23:27 am
This is SO useful for soap makers who are starting out. PLEASE add more!
Reply
Your comment will be posted after it is approved.
Leave a Reply. |
Peak Soap
The Original Bakewell Soap Co.
Contact : [email protected] | Copyright 2023 © P e a k S o a p Ltd.
P e a k S o a p™ Ltd. is registered in the United Kingdom | Office : Diamond House, Water Street, Bakewell, DE45 1EW | Company No. 11684582
P e a k S o a p™ Ltd. is registered in the United Kingdom | Office : Diamond House, Water Street, Bakewell, DE45 1EW | Company No. 11684582